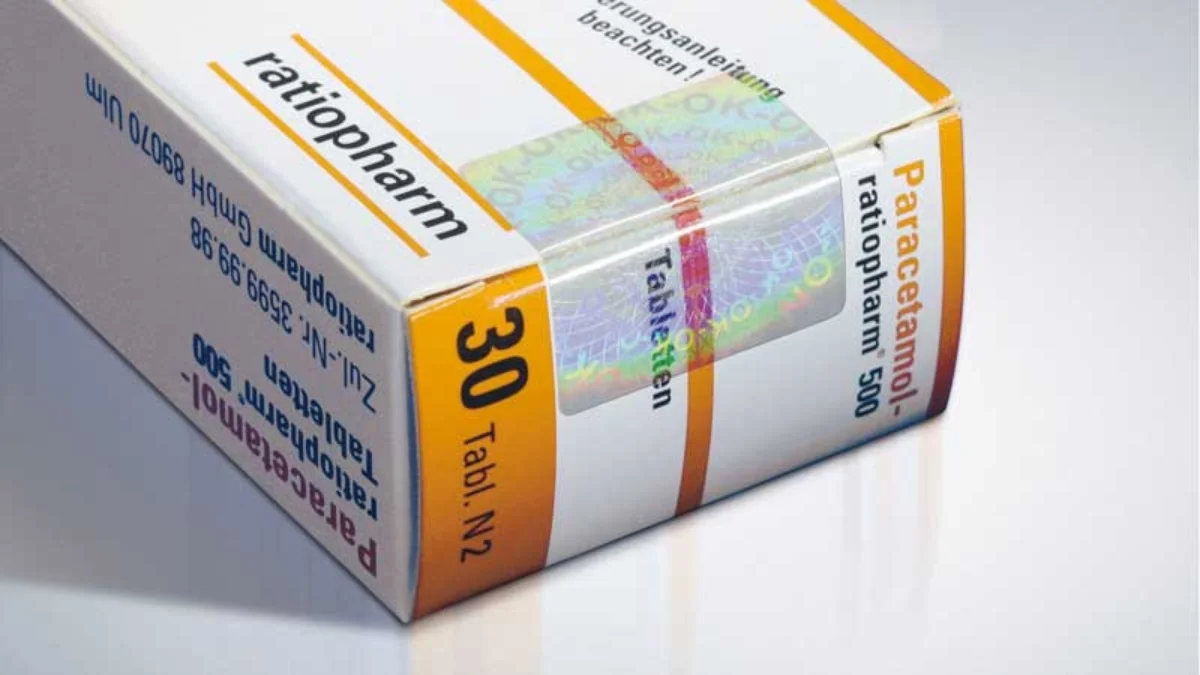
The pharmaceutical industry stands at the frontline of trust, where safety, authenticity, and transparency are non-negotiable. With India’s pharma market projected to reach $65 billion by 2024 and counterfeiting losses estimated at ₹4,000+ crores annually, pharmaceutical security labels India solutions have become critical for brand protection and patient safety.
At Holosafe, we’ve invested years of research into patented anti-counterfeiting technologies that transform ordinary packaging into intelligent security systems, ensuring every medicine reaching consumers is authentic, safe, and tamper-proof.
The Growing Threat to Indian Pharmaceutical Industry
Counterfeiting Statistics That Demand Action
The Indian pharmaceutical sector faces unprecedented security challenges:
- 12-15% of global counterfeit drugs circulate through Indian markets
- ₹4,000+ crores annual losses due to pharmaceutical counterfeiting
- 25% increase in parallel trading affecting brand integrity
- Rising consumer awareness demanding authenticity verification
These numbers underscore why anti-counterfeiting packaging solutions are no longer optional—they’re essential for business survival and patient protection.
Beyond Traditional Packaging: The Security Imperative
Modern pharmaceutical packaging must serve dual purposes: protection and authentication. Traditional labels that focus solely on product information leave brands vulnerable to sophisticated counterfeiting operations that can replicate basic packaging elements with alarming accuracy.
This is where advanced holographic security labels pharma solutions become the difference between vulnerable products and fortified brand protection.
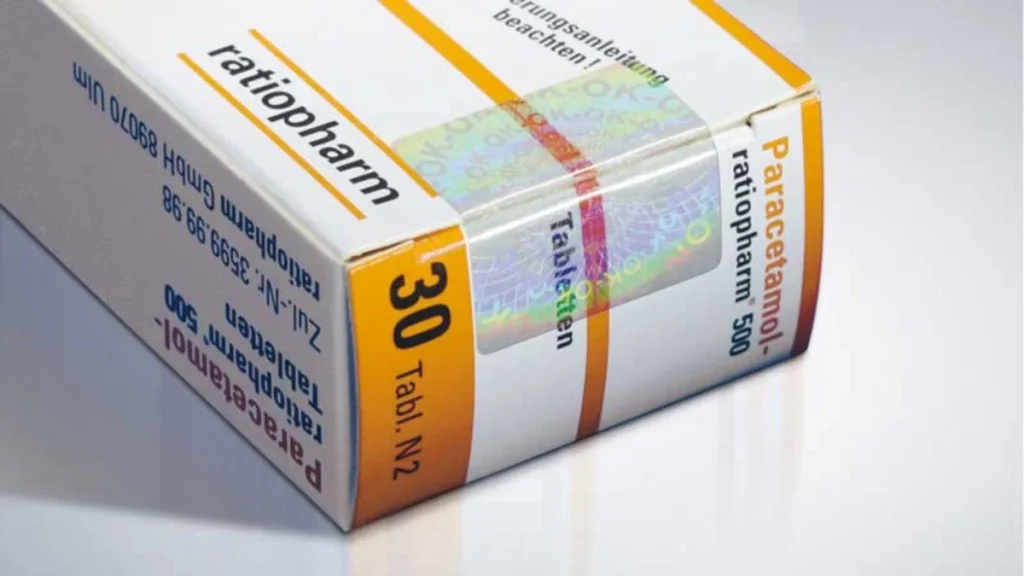
Holosafe’s Patented Label Technology for Pharmaceutical Security
Our pharmaceutical security labels go far beyond aesthetic enhancement. Each label functions as a multi-layered security ecosystem, integrating cutting-edge materials with intelligent design principles that make counterfeiting exponentially more difficult and expensive for fraudsters.
Advanced Material Engineering
Holosafe’s patented labeling solutions incorporate:
Substrate Innovation:
- Tamper-evident base materials that show clear signs of manipulation
- Chemical-resistant substrates suitable for pharmaceutical storage conditions
- Temperature-stable compositions for cold-chain and tropical climate durability
- Adhesive formulations that prevent clean removal and reapplication
Security Integration:
- Multi-layer construction with embedded security elements
- Optical security features visible under different lighting conditions
- Tactile elements providing physical verification points
- Machine-readable components for automated authentication
Seamless Manufacturing Integration
Our security labels integrate effortlessly into existing pharmaceutical packaging lines without requiring major equipment modifications or workflow disruptions. This practical approach ensures that enhanced security doesn’t compromise production efficiency or increase operational complexity.
Anti-Clonable QR Codes: Revolutionary Digital Authentication
One of the most pressing challenges facing the pharmaceutical industry is the cloning of standard QR codes used for track-and-trace compliance. Criminal organizations have developed sophisticated methods to replicate basic QR codes, undermining digital authentication systems.

The Cloning Problem
Standard QR codes present multiple vulnerabilities:
- Static data structures that can be reverse-engineered
- Predictable serial patterns enabling systematic counterfeiting
- Limited encryption making code duplication straightforward
- No real-time validation allowing offline code generation
Holosafe’s Anti-Clonable QR Solution
Our QR code authentication pharma system addresses these vulnerabilities through:
Advanced Cryptographic Protection:
- Dynamic encryption algorithms that change with each code generation
- Blockchain-backed verification ensuring immutable authenticity records
- Multi-factor authentication requiring multiple validation checkpoints
- Real-time server communication for instant authenticity confirmation
Unique Serialization Architecture:
- Non-linear serial patterns preventing systematic code prediction
- Embedded manufacturing metadata linking codes to specific production batches
- Geographic validation restricting code functionality to authorized regions
- Time-sensitive activation limiting code validity to appropriate distribution windows
User-Friendly Verification:
- Simple smartphone scanning requiring no special apps or training
- Instant authenticity confirmation with clear pass/fail indicators
- Multi-language support for diverse Indian market requirements
- Offline verification backup using visual security elements
Comprehensive Substrate Portfolio for Diverse Pharmaceutical Applications
Every pharmaceutical product presents unique packaging challenges, from sensitive biologics requiring cold-chain storage to generic tablets distributed in tropical climates. Holosafe provides tamper evident pharmaceutical labels across an extensive range of substrates to match these varied requirements.
Paper-Based Security Solutions
Cost-Effective Compliance Labels:
- Security paper formulations with embedded fibers and watermarks
- Chemical-reactive inks that change color when exposed to tampering attempts
- Microprinting technology creating verification elements invisible to casual observation
- Sequential numbering systems enabling batch-level tracking and authentication
Applications: Generic medications, over-the-counter products, compliance labeling
Advanced Filmic Security Labels
High-Performance Protection:
- Moisture barrier properties protecting labels in humid storage conditions
- Chemical resistance maintaining integrity when exposed to cleaning agents
- UV stability preventing degradation under fluorescent lighting
- Tear resistance ensuring labels remain intact throughout distribution
Applications: Premium pharmaceuticals, export products, hospital-grade medications
Tamper-Evident Foil Systems
Maximum Security Applications:
- Void-revealing adhesives that show “VOID” or custom messages when removal is attempted
- Destructible substrates that fragment when tampering occurs
- Multi-layer construction requiring complete destruction for access
- Forensic markers enabling post-tampering investigation and analysis
Applications: High-value medications, controlled substances, critical care pharmaceuticals
Specialty Substrates for Challenging Environments
Cold-Chain Compatible Materials:
- Low-temperature adhesives maintaining bond strength at -20°C to +25°C
- Condensation-resistant surfaces preventing label degradation in temperature transitions
- Flexible substrates accommodating packaging expansion and contraction
- Regulatory compliance meeting pharmaceutical cold storage requirements
High-Temperature Stability:
- Heat-resistant compositions maintaining integrity up to 80°C
- Tropical climate optimization for Indian subcontinent distribution
- Accelerated aging resistance ensuring long-term label performance
- Steam sterilization compatibility for hospital and clinical applications
Multi-Layer Security: Overt and Covert Protection Features
Effective pharmaceutical security requires multiple authentication layers that address different verification scenarios—from consumer-level authenticity checking to forensic investigation capabilities.
Overt Security Features (Consumer-Visible)
Holographic Elements:
- 3D holographic images providing immediate visual authentication
- Color-shifting patterns that change appearance based on viewing angle
- Micro-text integration combining holography with readable security information
- Brand-specific hologram designs creating unique visual identification
Tactile Security Elements:
- Embossed textures providing physical verification through touch
- Raised printing effects creating distinctive surface patterns
- Braille-compatible features ensuring accessibility for visually impaired users
- Temperature-sensitive materials that respond to human touch
Color-Change Technologies:
- Thermochromic inks that shift colors with temperature variations
- Photochromic elements responding to UV light exposure
- Solvent-reactive indicators revealing tampering attempts through color changes
- pH-sensitive materials detecting chemical exposure
Covert Security Features (Investigation-Level)
Microscopic Authentication:
- Micro-printing elements requiring magnification for visibility
- Nano-scale security features detectable only with specialized equipment
- Hidden text patterns embedded within apparent design elements
- Forensic-grade markers enabling detailed authenticity analysis
Invisible Ink Systems:
- UV-fluorescent materials visible only under ultraviolet illumination
- Infrared-responsive inks detectable with specialized scanning equipment
- Magnetic particle integration enabling machine-readable verification
- Chemical markers providing laboratory-level authentication capabilities
Digital Watermarking:
- Invisible digital codes embedded within label graphics
- Machine-readable patterns detectable through smartphone apps
- Blockchain integration linking physical labels to digital authenticity records
- Supply chain tracking enabling complete product journey documentation
Industry-Specific Applications and Case Studies
Generic Pharmaceutical Protection
Challenge: Generic manufacturers face unique counterfeiting pressures due to lower profit margins and high-volume distribution requirements.
Solution: Cost-effective security paper labels with embedded authentication features and sequential numbering systems.
Results:
- 87% reduction in reported counterfeiting incidents
- Enhanced distributor confidence and market acceptance
- Improved regulatory compliance and audit performance
- Strengthened brand reputation in competitive generic markets
Export Pharmaceutical Security
Challenge: Indian pharmaceutical exports face stringent international authentication requirements and cross-border counterfeiting risks.
Solution: Multi-substrate security labels combining holographic elements with anti-clonable QR codes and temperature-resistant materials.
Results:
- 100% compliance with destination country authentication requirements
- Zero reported counterfeiting incidents in key export markets
- Enhanced international distributor relationships
- Premium pricing justification through proven security measures
High-Value Medication Protection
Challenge: Specialty medications and biologics require maximum security due to high value and critical patient impact.
Solution: Comprehensive security systems featuring tamper-evident foils, covert authentication elements, and blockchain-integrated QR codes.
Results:
- Complete elimination of counterfeiting in protected product lines
- Enhanced patient safety and treatment efficacy
- Strengthened relationships with hospitals and specialty pharmacy networks
- Improved insurance coverage and reimbursement rates
Regulatory Compliance and Industry Standards
Indian Regulatory Framework
Drug and Cosmetic Act Compliance:
- Track and trace requirements mandated for pharmaceutical distribution
- Authentication technology standards specified by Central Drug Standard Control Organization (CDSCO)
- Import/export documentation requiring authenticity verification
- State-level enforcement driving demand for robust security measures
International Standards Integration
WHO Guidelines:
- Global surveillance systems requiring compatible authentication technologies
- Pharmacovigilance integration linking security features with safety monitoring
- Quality assurance protocols demanding verifiable authentication methods
- International cooperation frameworks facilitating cross-border authenticity verification
FDA and EMA Requirements:
- Serialization mandates requiring unique product identification
- Supply chain security demanding end-to-end traceability
- Import verification requiring robust authentication for pharmaceutical imports
- Post-market surveillance utilizing security features for product monitoring
Technology Integration and Future-Proofing
IoT and Smart Packaging Integration
Connected Authentication:
- NFC integration enabling smartphone-based instant verification
- Sensor integration monitoring storage conditions and product integrity
- Real-time tracking providing continuous supply chain visibility
- Automated alerts notifying stakeholders of potential security breaches
Artificial Intelligence Enhancement
Machine Learning Applications:
- Pattern recognition identifying sophisticated counterfeiting attempts
- Predictive analytics anticipating security threats and vulnerabilities
- Quality control automation ensuring consistent security feature application
- Supply chain optimization improving distribution efficiency while maintaining security
Blockchain Integration
Immutable Authentication Records:
- Distributed verification eliminating single points of failure
- Transparent audit trails providing complete product journey documentation
- Smart contract automation streamlining authentication and verification processes
- Cross-industry collaboration enabling secure information sharing among stakeholders
Implementation Strategy and Support
Phased Implementation Approach
Phase 1: Assessment and Planning
- Comprehensive security audit of existing packaging systems
- Risk assessment identifying vulnerabilities and priorities
- Custom solution design matching specific product requirements
- Regulatory compliance verification and documentation
Phase 2: Pilot Program Deployment
- Limited product line implementation for testing and validation
- Performance monitoring and feedback collection
- Process optimization based on real-world operational data
- Stakeholder training and change management support
Phase 3: Full-Scale Rollout
- Complete product portfolio protection implementation
- Supply chain partner integration and training
- Market communication and customer education programs
- Ongoing monitoring and continuous improvement protocols
Comprehensive Support Services
Technical Support:
- 24/7 helpdesk for authentication verification and troubleshooting
- Regular software updates and security enhancement deployments
- Integration support for existing packaging and distribution systems
- Custom training programs for internal teams and supply chain partners
Regulatory Assistance:
- Compliance documentation and regulatory filing support
- International standards certification and validation
- Audit preparation and regulatory inspection assistance
- Ongoing regulatory monitoring and update communications
Cost-Benefit Analysis for Pharmaceutical Companies
Investment Considerations
Implementation Costs:
- Label material costs: typically 15-30% premium over standard labels
- Integration expenses: minimal for most existing packaging lines
- Training requirements: 2-3 days for operational team certification
- Ongoing support: included in comprehensive service packages
Return on Investment:
- Brand protection value: Prevention of counterfeiting incidents worth ₹10-50 lakhs per product line
- Market premium: 8-15% price premium justification through proven authenticity
- Insurance benefits: Reduced liability and coverage costs through enhanced security
- Regulatory advantages: Streamlined approval processes and compliance maintenance
Risk Mitigation Benefits
Quantifiable Risk Reduction:
- 95%+ reduction in successful counterfeiting attempts
- Elimination of parallel import concerns through geographic authentication
- Enhanced supply chain visibility reducing diversion risks
- Improved customer confidence and brand loyalty metrics
Future Trends in Pharmaceutical Security
Emerging Technologies
Next-Generation Authentication:
- DNA-based markers providing ultimate authentication security
- Quantum encryption offering theoretically unbreakable security codes
- Biometric integration linking products to authorized handlers
- AI-powered verification enabling autonomous authentication systems
Regulatory Evolution
Anticipated Requirements:
- Global serialization standards requiring universal compatibility
- Real-time reporting mandates demanding instant authenticity updates
- Consumer verification access enabling patient-level authentication
- Cross-border cooperation facilitating international security coordination
Market Transformation
Industry Shifts:
- Security-first packaging becoming standard rather than premium feature
- Consumer-driven authentication with patient-initiated verification
- Supply chain transparency as competitive advantage
- Technology partnerships between pharmaceutical and security companies
Contact Holosafe for Pharmaceutical Security Solutions
Ready to protect your pharmaceutical products with advanced security labeling technology? Our team of anti-counterfeiting experts is available to assess your specific requirements and design custom solutions that match your product portfolio, distribution channels, and security objectives.
Get Started Today:
- Free Security Assessment: Comprehensive evaluation of your current packaging vulnerabilities
- Custom Solution Design: Tailored security features matching your specific requirements
- Pilot Program Options: Risk-free testing with limited product implementations
- Regulatory Support: Complete compliance assistance and documentation